Giving adjuvant Xeloda to Biliary cancer was and is crazy
The trial was negative, and 2 other trials are negative-- what the hell are we doing?
Recently, I took care of a patient with resected biliary tree cancer who received adjuvant Xeloda. He was back in the hospital because the cancer had returned. We broke the unfortunate news. He told me how hard the prior therapy was for him, and how much he hoped it had worked.
For me, I couldn’t get past the simple fact that his doctor had given him chemotherapy that had FAILED in a randomized study— it could only add toxicity and cost, and no one had told him that the decision to prescribe the drug was a gamble— there was no clear evidence of benefit.
Our new paper explores this issue
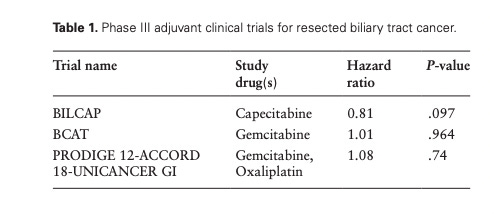
It turns out there are 3 adjuvant studies in resected biliary cancer and these are the results. All three are negative
BilCAP is the closest to positive, and many have accepted the p value, arguing that there is only a 10% chance of seeing a result this extreme or more, under the null hypothesis that it doesn’t work. But this is wrong.
There is closer to a 1 in 3 chance of a seeing a BILCAP like result under the null hypothesis that none of these drugs work in 1 or more trial— because you are running 3 trials!
That is unacceptably and crazy high. It would be like accepting p values of .33 as nominally significant and acting upon it.
Bottom line: BILCAP is not and should not be practice changing. It should be replicated, at most.
We conclude our article with this.
The full PDF is attached for subscribers.
Subscribe to this newsletter for drug development— and not COVID— commentary.