How many people benefit from CAR-T?
We talk about it all the time but is the benefit commensurate?
Chimeric antigen receptor (CAR)-T therapies are a group of novel cancer treatment options that have generated a lot of excitement because of high response rates and durable remissions in some individuals. Currently, they are only approved for hematologic indications, but they are being tested in patients with solid tumors.
Using methodology we previously used for other types of cancer therapy, we estimated the number and percentage of people with advanced or metastatic cancer in the US who may be eligible for or respond to CAR-T therapies – in other words, we wanted to see how many people may benefit from these novel therapies.
Using the American Cancer Society’s Cancer Facts and Figures, we used the number of deaths for cancers with a CAR-T therapy approval as a stand-in for the number of people who are eligible. We assumed this because CAR-T therapies are often given in later lines of therapy when other treatment options for patients have been unsuccessfully exhausted. From the US FDA labels for each approved CAR-T therapy, we identified the reported response rate and multiplied it by the number of people who died from the respective cancer type. We then divided the number of people who responded by the total number of deaths to get a percentage of response.
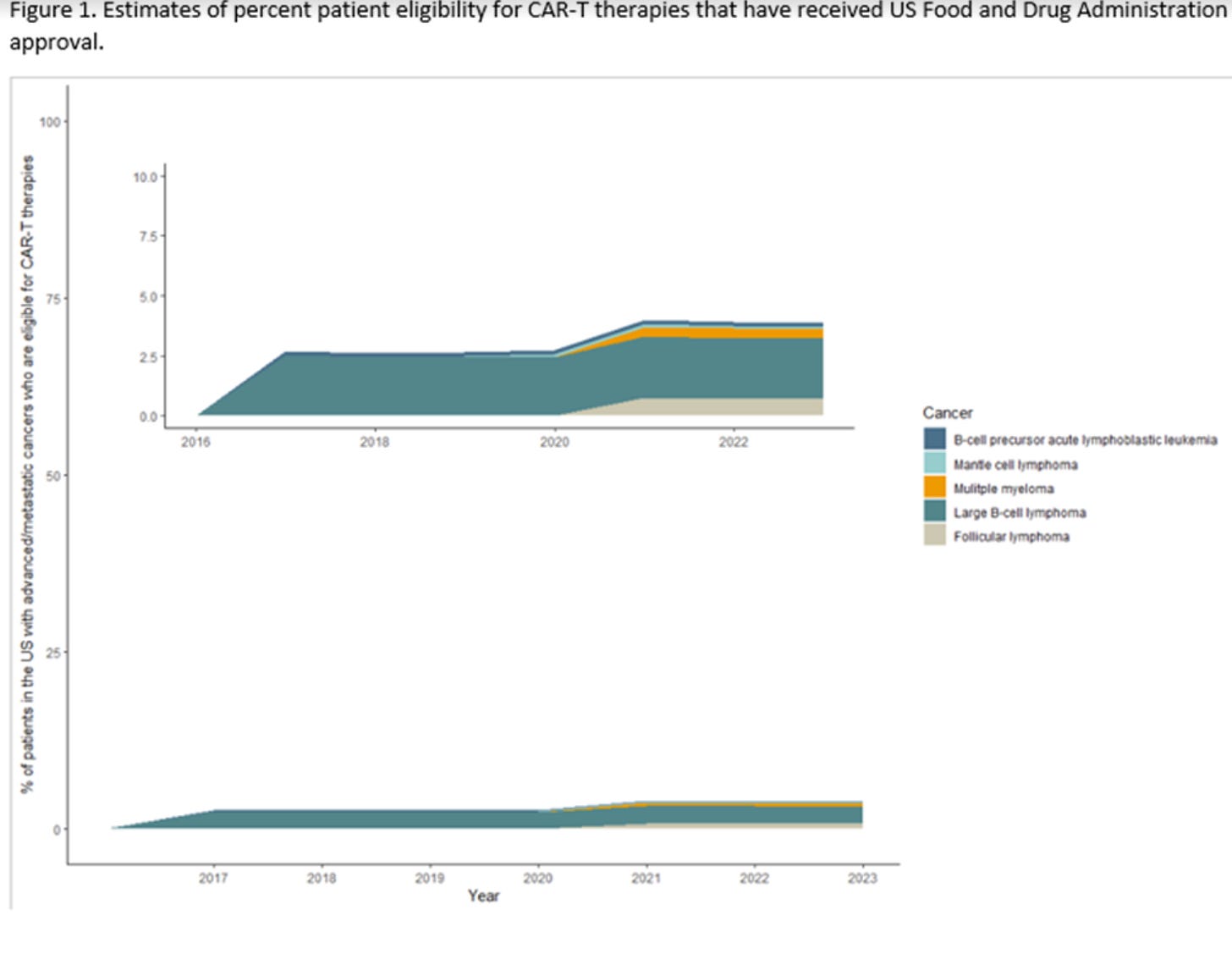
Six CAR-T therapies were approved for 13 indications. The percentage of people with advanced or metastatic cancers who were eligible for CAR-T therapies was estimated to be 2.7% (95% CI: 2.6% to 2.7%) in 2017, when the first CAR-T was approved, and 3.9% (95% CI: 3.8% to 3.9%) in 2023. Using the reported response rates in the FDA package inserts, the percentage of people with advanced or metastatic cancer who had the potential to respond to CAR-T therapies was 2.0% (95% CI: 1.9% to 2.0%) in 2017 and 3.4% (95% CI: 3.4% to 3.5%) in 2023. In 2023, the tumor that had the highest contribution to both eligibility and response was large B-cell lymphoma, and the lowest contribution was for mantle cell lymphoma.
While these treatments have been successfully used in some patients, the estimated percentage of both eligibility and response are generally low. Moreover, because these treatments cost more than other types of cancer therapies with a high price tag, the cost-effectiveness of these therapies— especially when they aren’t curative like myeloma— may be questionable.