Neoadjuvant immunotherapy in patients with melanoma... let's get ready for ASCO-2024 plenary !
A re-analysis of the SWOG S1801 trial (neoadjuvant pembrolizumab versus adjuvant only), and more...
In this ASCO 2024 series, we provide background in expectation of the results to be presented during the upcoming ASCO 2024 meeting, which will take place between 31th May and 4th June. The annual congress of the American Society of Clinical Oncology is a key event in oncology. Each year, it attracts more than 30,000 attendees in Chicago.
Patients with resected melanoma are often proposed adjuvant immunotherapy, i.e. AFTER surgical removal, depending on the disease stage, based on DFS or EFS gains. Now, buyoed by successes in other tumor types where 3 immunotherapy drugs have been approved in the neoadjuvant settings – i.e BEFORE surgery – similar studies were run in melanoma.
The topic is “hot”! The first results of the NADINA phase 3 trial, testing 2 doses of ipilimumab and nivolumab before surgery, will be presented during the Plenary at ASCO 2024 in a few weeks!
What are the Pros and Cons of neoadjuvant strategies ? To make it short :
Theoretical benefits:
rapid tumor control
assessing tumor response based on the surgically removed specimen
enhanced tumor immunogenecity (with the tumor not yet removed)
Theoretical harms:
toxicity which can delay or preclude surgery
disease progression before surgery
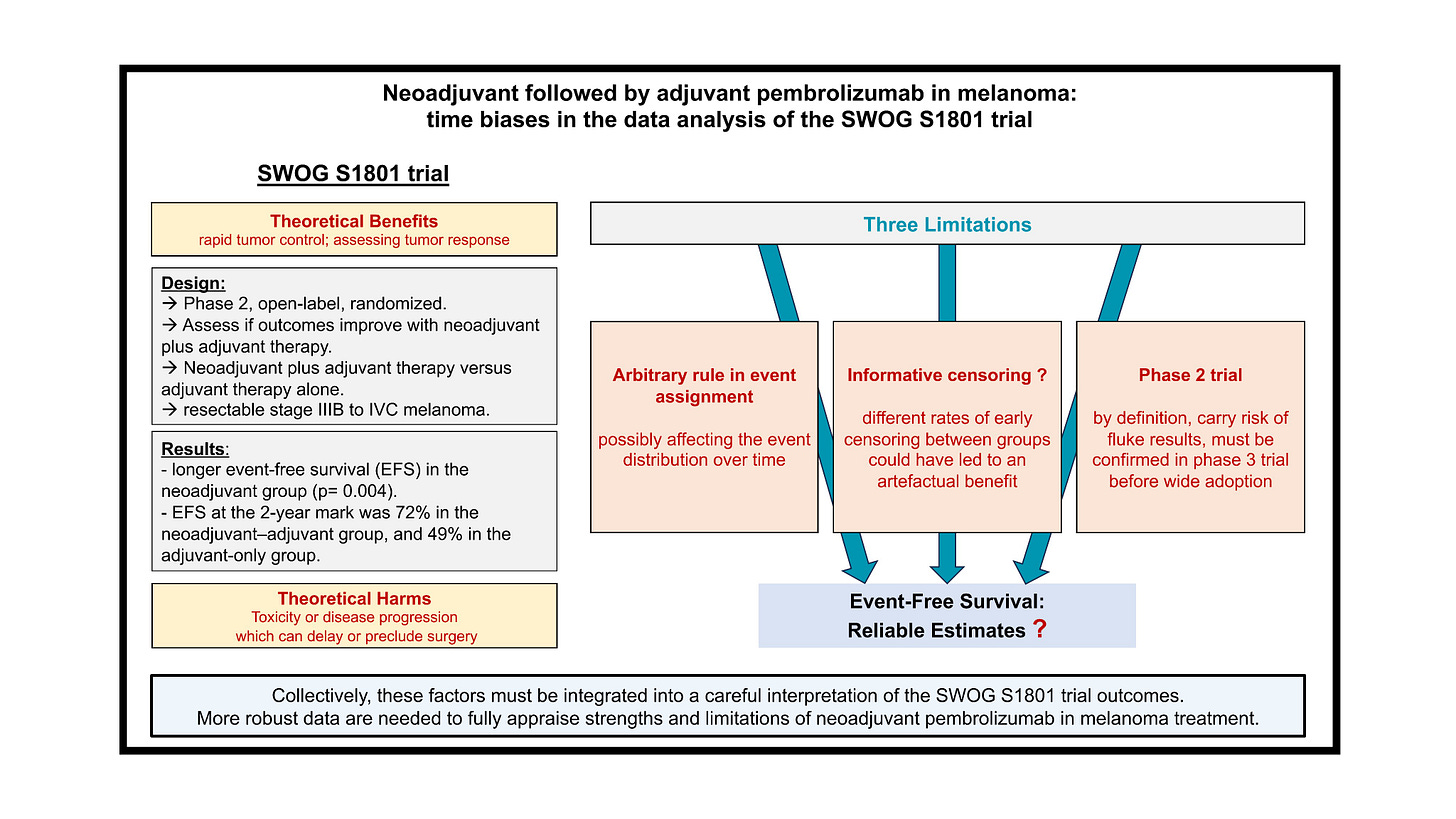
The SWOG S1801 trial - a bizarre rule introducing time biases.
In a recent paper, which is openly available here, we explored the results and limitations of the SWOG S1801 trial, which is another trial in the neoadjuvant setting.
This was a phase 2 trial enrolling patients with resectable stage III-IV melanoma, and randomizing patients between a “pembro-first” strategy (3 cycles of pembrolizumab followed by 15 cycles post-surgery) vs an “adjuvant only” strategy. The study was positive, with a longer event-free survival (EFS) in the neoadjuvant group (p= 0.004). EFS at the 2-year landmark was 72% in the neoadjuvant group, and 49% in the adjuvant. The trial was published in the NEJM creating enthusiam in the melanoma community. However, someting very unusual catches the eye when looking at the Kaplan-Meier plot, can you spot it?
Yes! The curves are abruptly dropping down in both groups at 12 weeks!
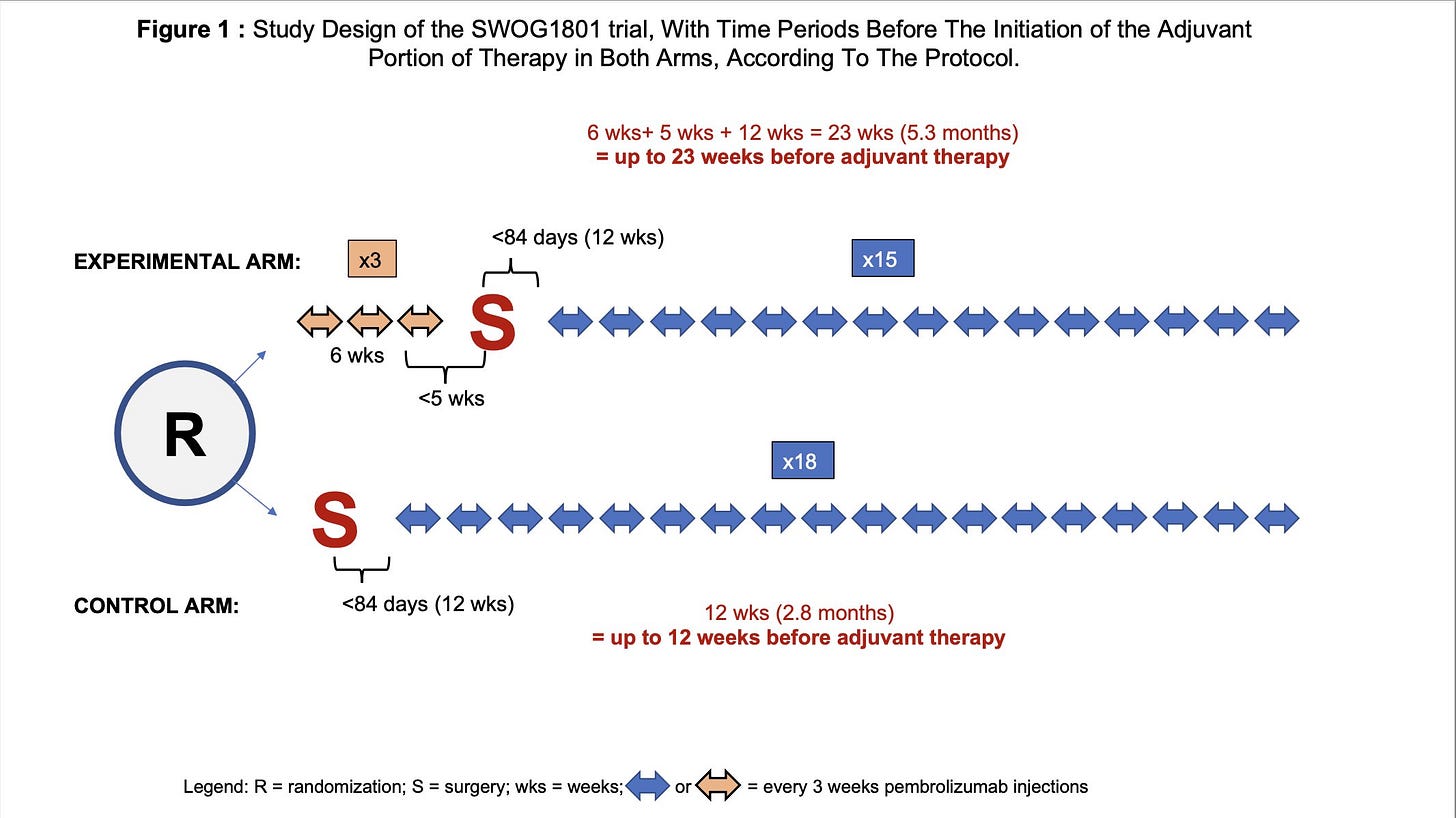
How is this possible? The explanation is an unusual rule which was applied. “On both arms, all participants who do not register for adjuvant therapy (for whatever reason) will be assigned the event time of 84 days.” (i.e 12 weeks).
However, if you look at the time-line in both groups (see Figure 1 below), the initiation of the adjuvant immunotherapy portion could occur up to 12 weeks in the control group, and up to 23 weeks in the experimental neoadjuvant group.
Problems with this rule:
it is masking the distribution of events over time, thus violating a core principle of the Kaplan-Meier methods
it introduces a “guarantee-time bias” for patients with early events: they will be attributed an event date of 12 weeks even if the real event date is day 2!
it attributes an earlier event date in patients having the event after 12 weeks, which could happen in the neoadjuvant arm up to 23 weeks.
Informative censoring ?
We estimated 17.5 % of patients were censored during the first 6 months in the experimental group and 11.9 % in the control arm. In other words, there were a significant and early imbalance in censoring rates, which is suggesting informative censoring. By running a conservative sensitivity analysis, modeling different fates for a fraction of censored patients over the first time-period, we showed the S1801 gains are fragile, with the statistical benefit litteraly fading away (Figure 2).
Following the principles of open-science, our analysis is openly available for reproduciblity here. We conclude that the results of S1801 remain fragile, and should ideally be confirmed in phase 3 trials.
What to look at in NADINA during ASCO 2024?
Now that you've observed what happened in the SWOG S1801 trial, it becomes clear just how crucial the number of censored patients in each group is, as well as understanding the reasons for their censoring.
Beyond the efficacy results (EFS is the primary endpoint), here are other key points to be followed during NADINA’s presentation:
how many patients could not undergo surgery?
what were the reasons: toxicity? disease progression?
are early censoring rates balanced between arms?
do we have long-term data on toxicity?
If you want to delve deeper in the topic, check out our full analysis of the S1801 trial !