"The landscape of checkpoint inhibitors in oncology" - a portrait of immunotherapy.
In a recent study, we aimed to provide an overview of pivotal clinical trial data supporting the marketing authorization of immune checkpoint inhibitors.
Checkpoint inhibitors have become common treatments for cancers, especially for some that have historically had poor prognosis because of limited effective treatment options (e.g., melanoma and lung cancer). Approvals for this class of drugs has been granted in most solid tumor types, including some for tumor-agnostic indications. Against the backdrop of increasing popularity for checkpoint inhibitors, there is little known about the totality of evidence for these types of cancer drugs.
The landscape
We set out to evaluate the collective benefit, in terms of overall survival and progression-free survival, of all approved checkpoint inhibitors. Our work was recently published in the European Journal of Cancer (openly available here).
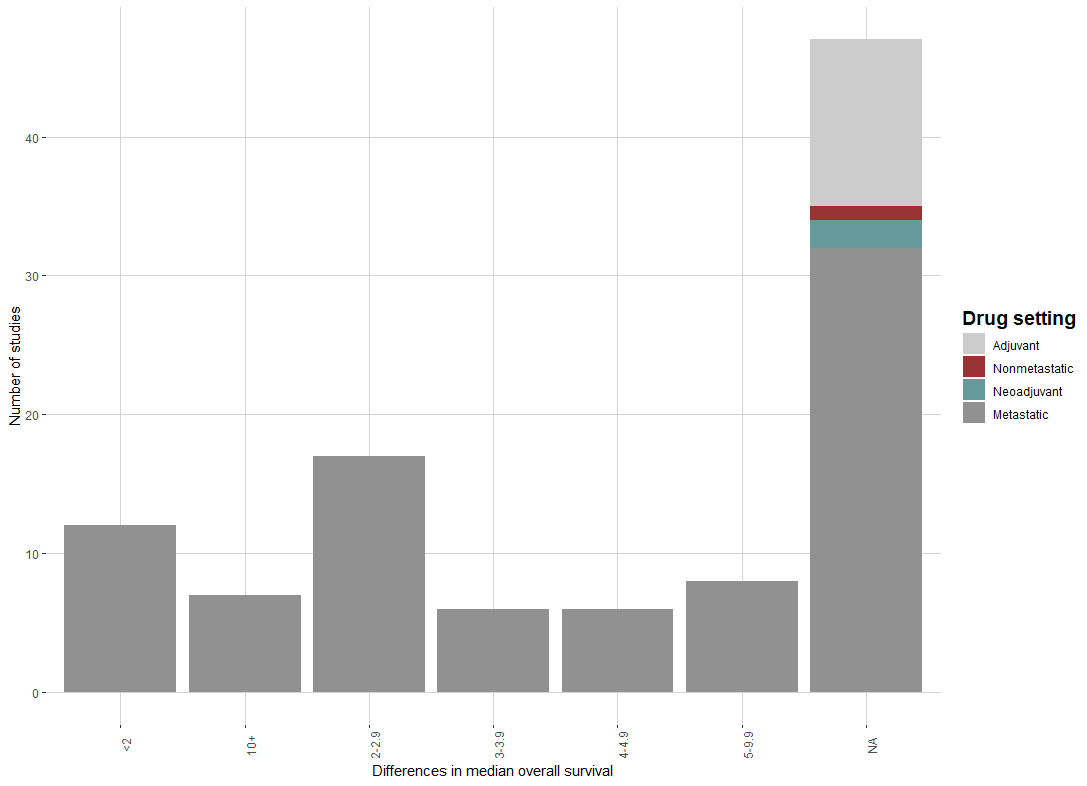
Of the 103 checkpoint inhibitor approvals, 56 (54.4%) reported overall survival information. Almost half of trials do not report survival data. Of those that do report, the median improvement in survival was a modest 2.8 months. The breakdown of improvement time in survival is shown in the figure.
Sixty-five (63.1%) of trials reported data on progression-free survival, and the median improvement was 0.9 months. Of those reporting data on progression-free survival, 20% had a difference of less than 0, meaning that patients in the control arm had a numerically longer time until tumor progression or death.
Another important finding is that the number of people in the control arm who crossed over to the investigative treatment was high (~20%). Crossover can bias the study results, making it difficult to reliably interpret efficacy findings. Moreover, crossover is only appropriate when the drug has shown to be beneficial when there is tumor progression.
Conclusion
The take-home message from our study show that checkpoint inhibitors have been tremendously valuable for some tumor indications (10-plus months of improved survival for 7% of indications), but for most indications, the improvement in survival is modest (less than 3-month benefit). Excitement for these drugs should be tempered to more realistically reflect the actual benefit from them. Read our full article here.





Good paper. Two questions:
1. Is there any evidence that there should have been some survival data in the papers which didn’t report it?
2. Are there any studies which appear to have been “buried” without publication?
Obviously my overarching point here is: are you sure you haven’t performed (in essence) a meta-analysis of a dataset curated to bias the drugs by a pharma-captured publication system?
Great work! Been looking for something like this. Looking forward to digging into the paper. A bit of confirmation bias here but those are the results I would have expected