The online BREAKING-ICE App© out in The Lancet Oncology !
Understanding Censoring in Kaplan-Meier Curves and Exploring Informative Censoring
The online BREAKING-ICE App© has been published recently in The Lancet Oncology, co-authored with Vinay Prasad and Alyson Haslam.
Informative censoring will no longer be a dry concept, you'll be able to visualize it live!
This freely accessible App - https://www.timotheeolivier-research.com/breaking-ice - best used on a computer, could revolutionize the way you approach censoring in survival analysis. Here is why.
What is censoring ?
In a clinical trial, each patient either experiences an event, or is censored if they have not experienced the event. This is why, on Kaplan-Meier curves, you will be seeing either tick marks or circles: they indicate patients that have been censored. What is usually expected in a trial is late censoring, and equal censoring.
Late censoring because patients who are still in a trial without having presenting the event will be censored at data cut-off.
Equal censoring because the rate of censoring should not be influenced by the arm to which the patients were allocated upon randomization. Censoring should be non-informative.
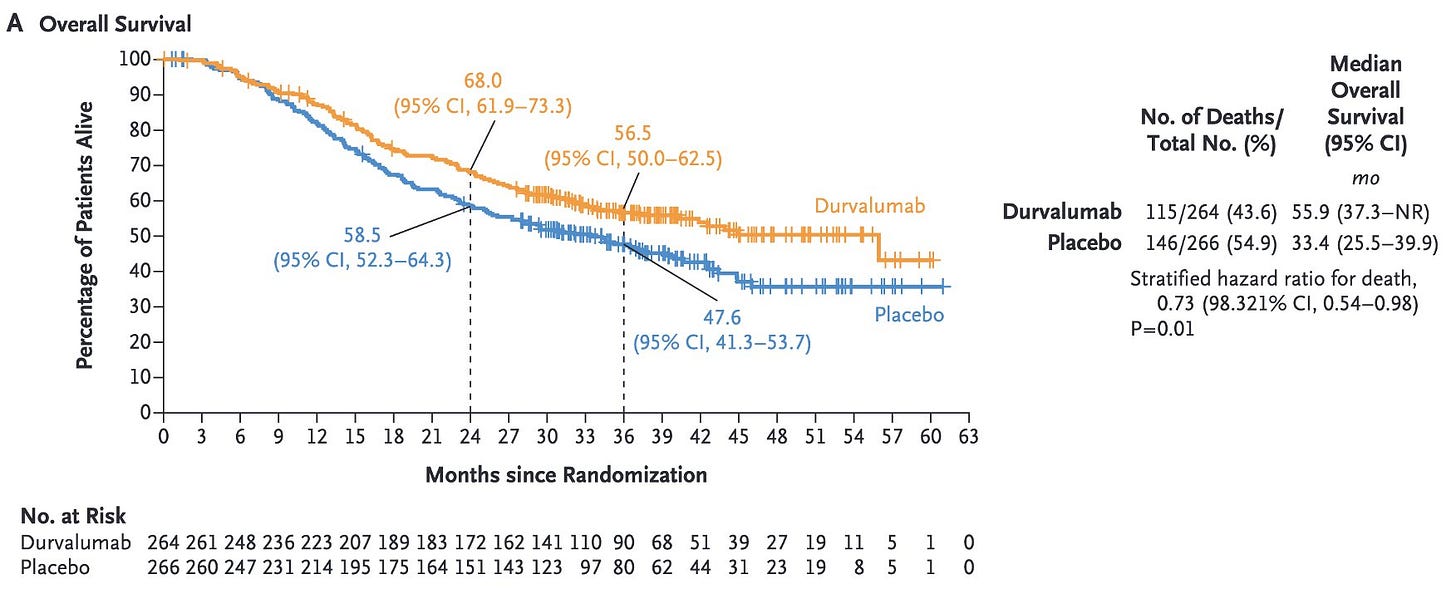
Let's have a look at the ADRIATIC trial (durvalumab after front-line therapy in limited-stage small-cell lung cancer).
In both curves, you can see tick marks mostly appearing after 27 months, probably due to censoring at data cut-off (median follow-up in both groups = 37.2 months), which is what is expected. However something is missing from the NEJM publication: where are the number of patients censored at each time point ?
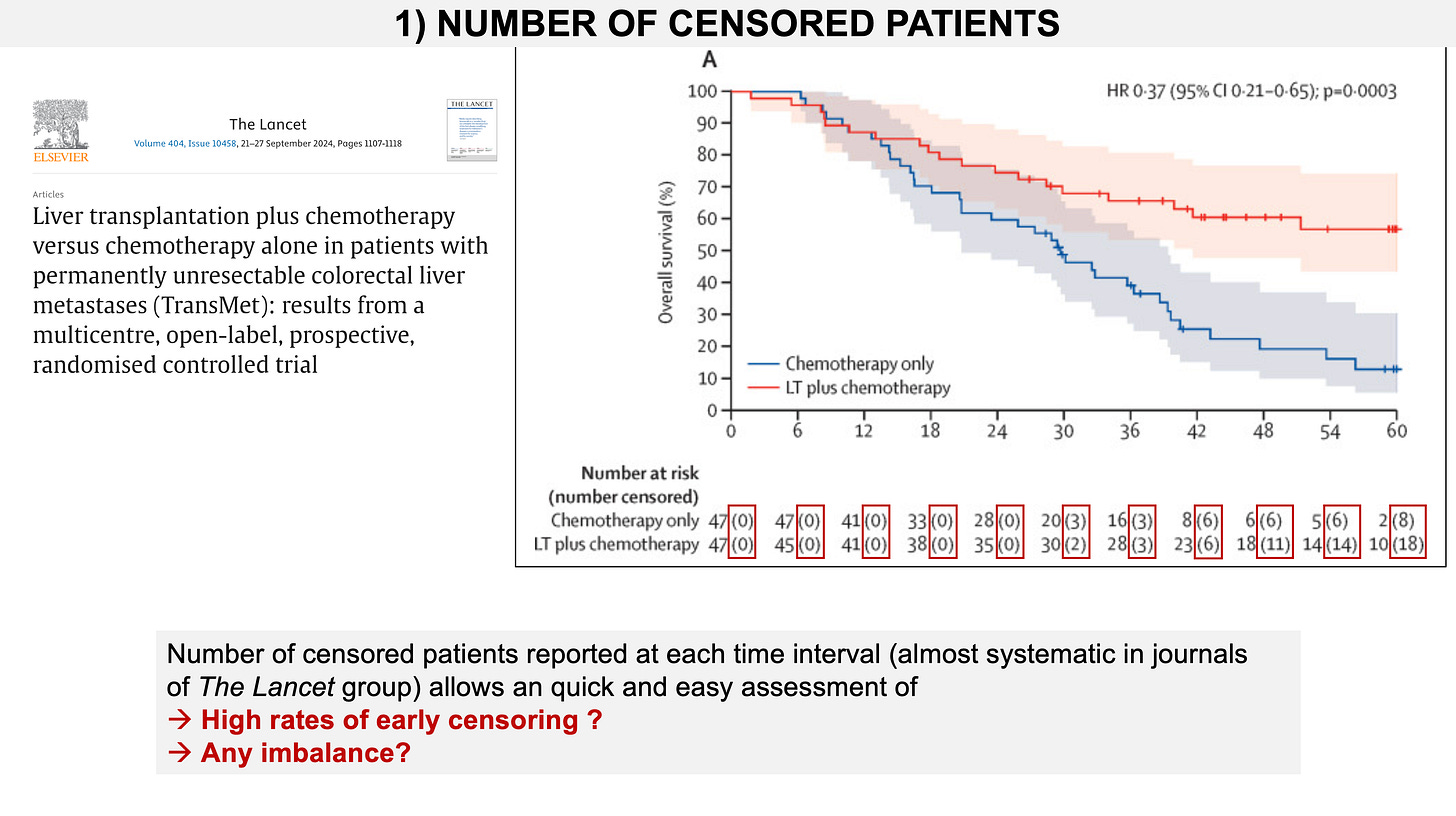
The Lancet approach, for years, is to report in brackets, after the number at risk, the number of patients that have been censored. Below is an example from the TransMet trial.
A first feature of the BREAKING-ICE App©
The App will provide estimates of censored numbers, following the Lancet approach, whenever those data are lacking in publications or presentations, which is unfortunately common.
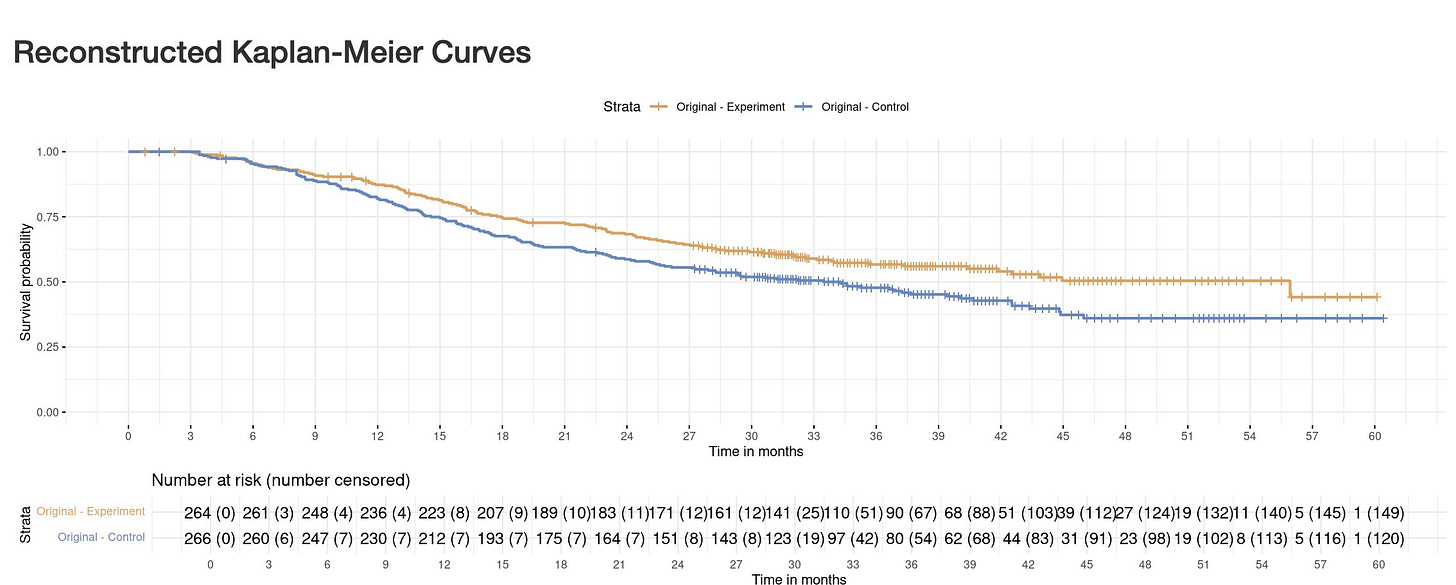
Let's have a look at ADRIATIC again. By using reconstructed Kaplan-Meier curves, the App allows you to estimate those numbers, which were not reported in the NEJM publication. You can also do it yourself: https://www.timotheeolivier-research.com/breaking-ice and in "Select Trial", choose, “ADRIATIC-OS”.
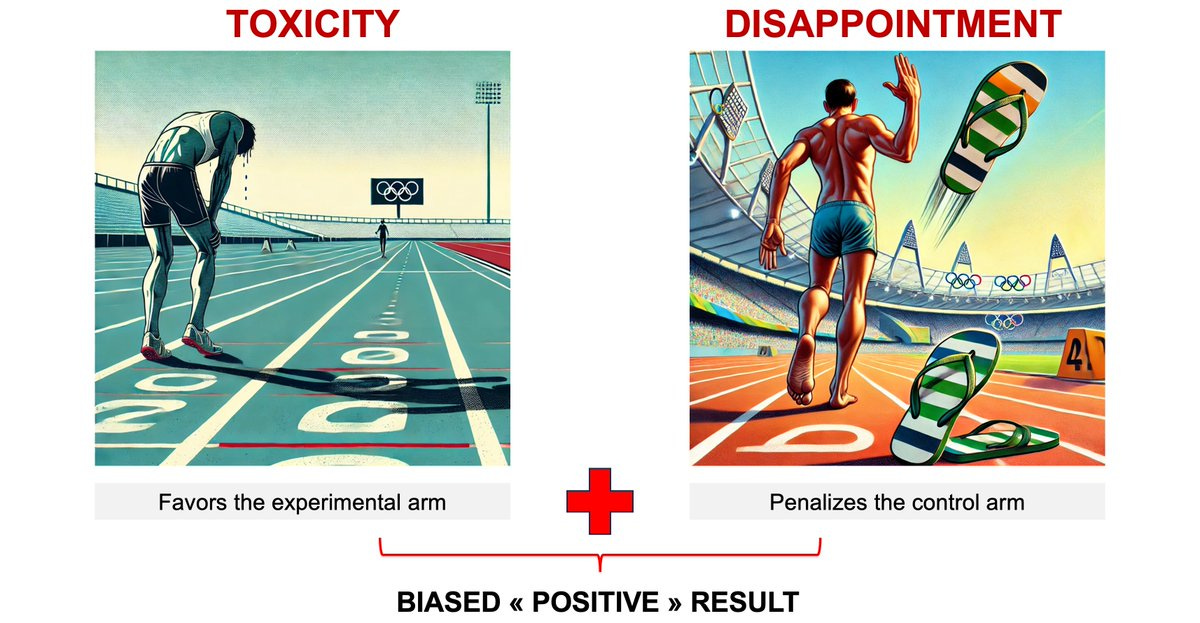
Two types of informative censoring: toxicity and disappointment.
When censoring is unbalanced, particularly early censoring, there may be reasons related to the allocated arm. How does this affect the survival estimates? Let's exemplify this with the two main reasons for informative censoring.
In a race with many runners, if you exclude those who dropped out before the finish, your average time will be skewed towards a faster pace. These analogies are not intended to trivialise being diagnosed with cancer, which is always a significant life event, and as a practising oncologist, I am fully aware of this. However, they can be very effective in understanding concepts.
When considering survival curves, individuals censored excessively might have been frailer and at higher risk of experiencing the event compared to those who remain in the study. If they had continued on the trial, they could have presented the event, but were censored before. This could retain individuals with a lower risk of the event, potentially favouring the experimental arm when it is more toxic.
Another common type of informative censoring is “disappointment-driven censoring”. Here again, let’s imagine you invite a group of people to run a race, and when they arrive, they find slippers… Some won’t accept to run such a race. If those who drop out are the ones who would have run faster, the average time will again be lower.
In a trial, particularly in open-label ones, where patients know the allocated arm, they may be disappointed. This typically happens in cases of a suboptimal control arm. In such situations, some patients will quit the study to seek other treatment opportunities outside the trial. A plausible hypothesis is that those leaving the trial are more connected, have more options, and are in better condition as compared to those remaining in a poor, suboptimal control arm. If this happens, the control arm will retain individuals at higher risk of presenting the event, and this will further penalize the control arm.
Both toxicity and disappointment can occur during a trial.
This is a key point, and a key feature in the BREAKING-ICE App© : you can modelize one or the other type of censoring in one or the other arm, depending assumptions in relation to the trial design.
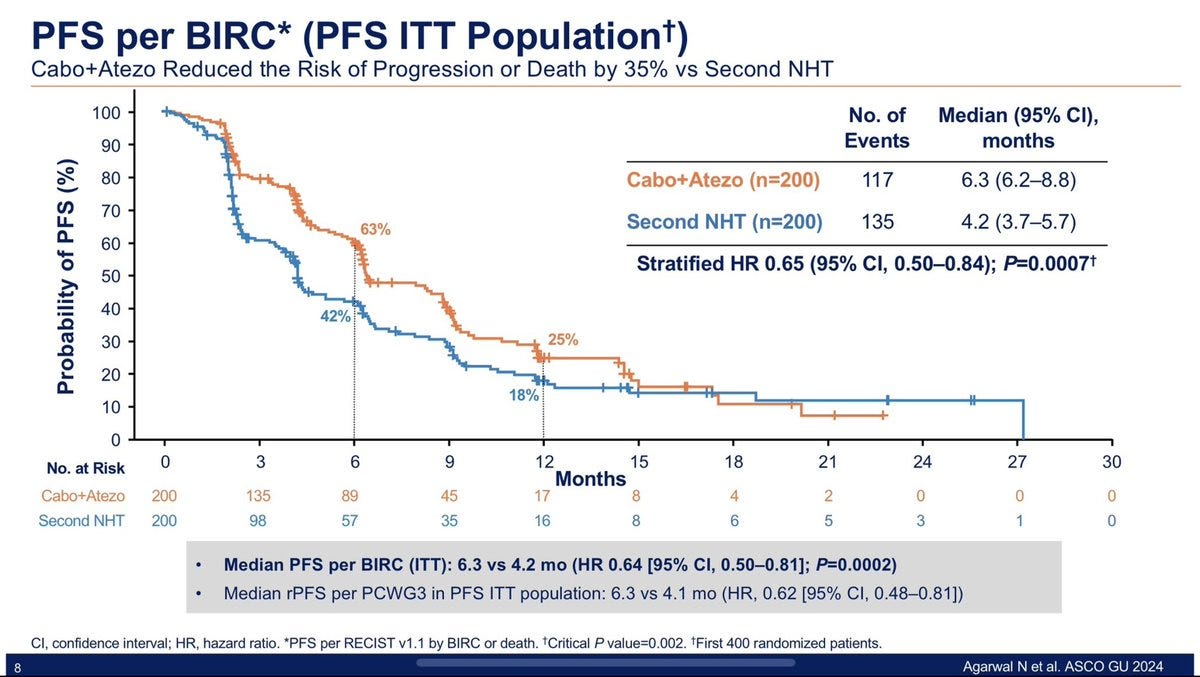
In the CONTACT-02 trial, patients were allocated to either a toxic experimental arm or a suboptimal control arm. Here is the trial design, followed by the primary endpoint results— a statistically significant Progression-Free Survival benefit.
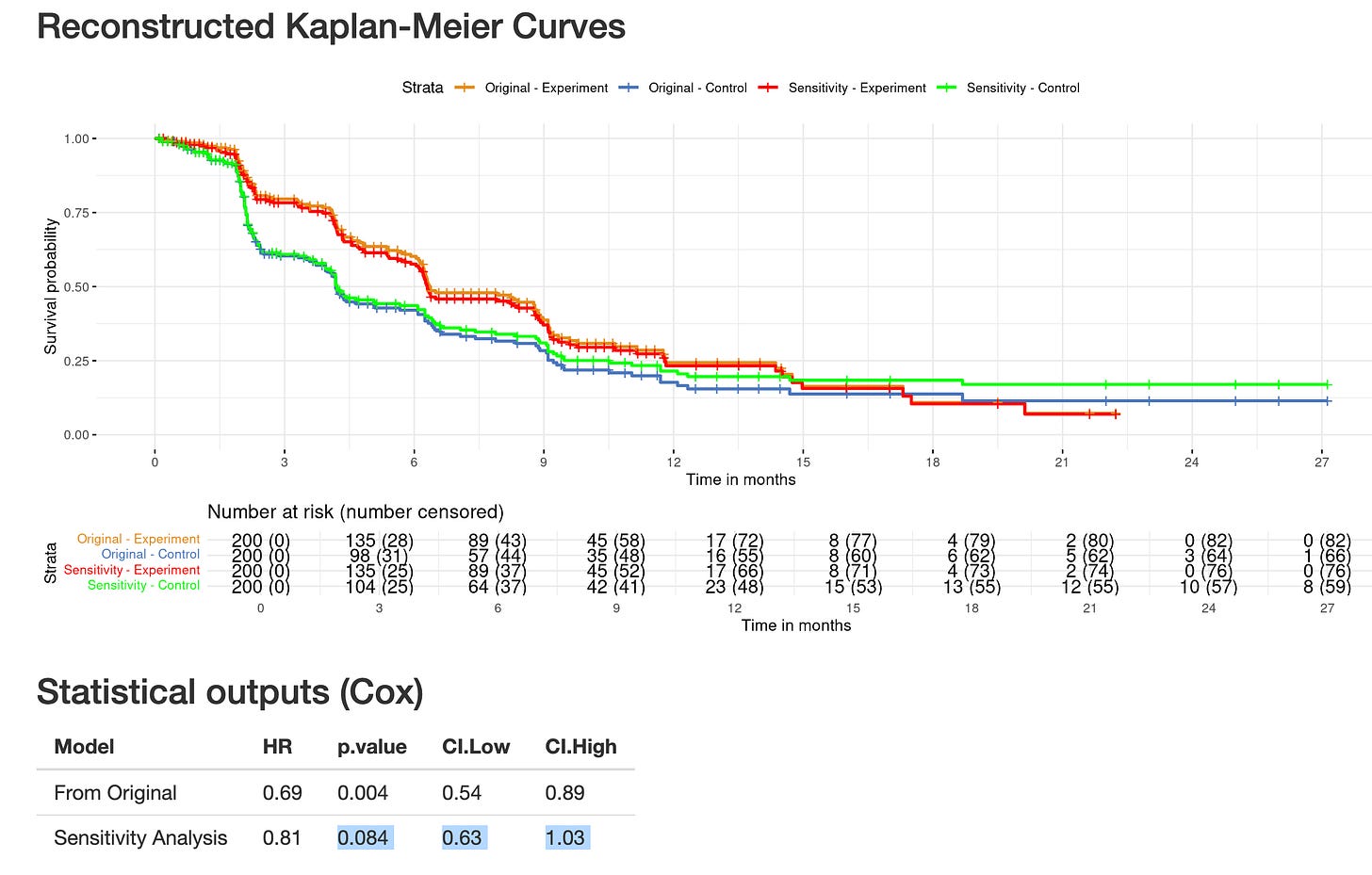
We performed a very conservative sensitivity analysis based on reconstructed Kaplan-Meier curves. We selected 15% of patients censored over the first six months in the experimental arm (more toxic), and hypothesized they would have presented the event instead of being censored. We then selected 15% of patients censored over the first six months in the control arm (disappointing), and hypothesized they would have stayed in the trial and not presented the event, and been late censored instead. With these two conservative and plausible assumptions, the curves are changing and the PFS benefit is no longer statistically significant.
This analysis has been published, and examplifies how censoring can still be informative even with equal rates of censoring, but different reasons for censoring (full article available here).
The BREAKING-ICE App©: 3 features.
The App uses synthetic individual patient data (IPD) from published Kaplan-Meier curves. One advantage is that there is a set of trials already available in the App, and many others will come! So there is no need to digitize curves yourself (which you can also do, of course).
Gives you estimates of censored patients at each time interval (including in a table below the curves)
This allows you to perform sensitivity analyses and visualise the live impact on the curves.
The statistical output of your analysis is also available.
Informative censoring is no more a dry concept. Use the App, if you are interested in a trial to be digitized, feel free to contact me. There is also a concise step-by-step tutorial here : https://www.timotheeolivier-research.com/breaking-ice-tutorial. The Lancet Oncology publication is also available here.
You can find an example of the sensitivity analyses I ran with the BREAKING-ICE App© in Part 1 of my CHALLENGE-trial analysis. This will also be useful for Part 2 – stay tuned!







One thing I don’t understand is why patients coming off drug due to toxicity need to be censored.they should still be followed on study for relapse/ survival .