DESTINY-Breast06 : why I hope there won't be a standing ovation at ASCO-2024.
Trastuzumab deruxtecan as first-line chemotherapy in Hormone-Receptor positive, HER2-low, metastatic breast cancer.
In this ASCO 2024 series, we provide background in expectation of the results to be presented during the upcoming ASCO 2024 meeting, which will take place between 31th May and 4th June. The annual congress of the American Society of Clinical Oncology is a key event in oncology. Each year, it attracts more than 30,000 attendees in Chicago.
In the last “Friday’s Gem”, we presented the E1193 trial, published in 2003, conducted in the first-line setting of patients with metastatic breast cancer, where the lack of survival or quality of life benefit were driving the main conclusions.
The DESTINY-Breast06 trial results will be presented at the upcoming ASCO, and the results are already presented as potentially practice-changing. Let’s review why I think the results should be taken with a big grain of salt.
DESTINY-Breast06
The study was an open-label, phase 3 trial, and enrolled patients with HER2-low (not HER2 positive nor HER2 negative, but more on this later) metastatic breast cancer. Patients should have received at least 1 previous line of endocrine therapy, and no prior chemotherapy in the metastatic setting.
Patients were randomized in two groups:
trastuzumab deruxtecan, an antibody-drug conjugate, meaning an antibody with a payload of chemotherapy.
investigator’s choice chemotherapy (also referred as “standard of care”)
PFS = primary endpoint, OS = secondary.
“Standard-of-care” chemotherapy ?
In a previous work of ours, we showed that physicians' or investigators' choices were “illusory choices” and restricted in 85% of cases. The problem arises when such restrictions prevent important options from being used, penalizing the control arm.
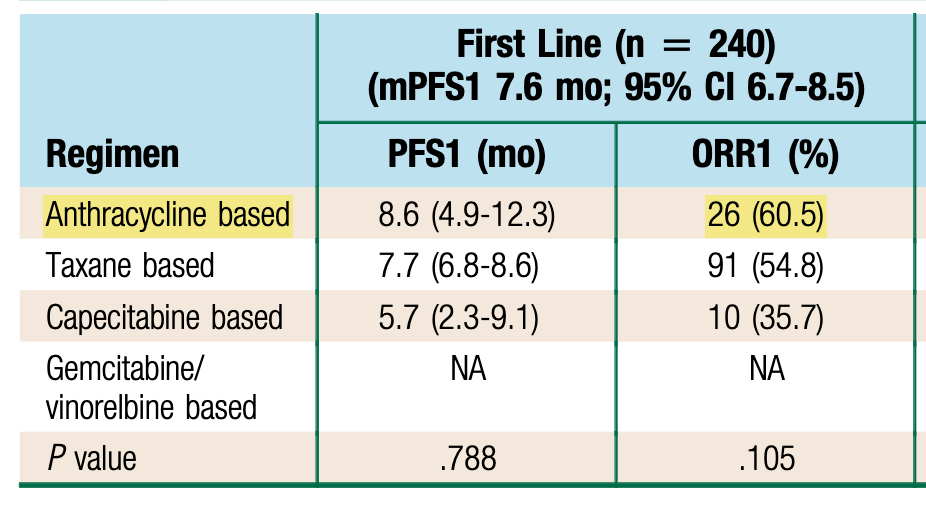
In the DESTINY-Breast06 trial, the control arm, also refered as “standard-of-care”, is a choice between capecitabine, paclitaxel and nabpaclitaxel… Is something missing here?
Where Have the Anthracyclines Gone?
Anthracyclines are a major therapeutic class in breast cancer treatment, yet this option is missing in the control arm of DESTINY-Breast06. This is concerning, as anthracycline-based therapy is one of the most active drug in this setting.
Many other options could be used, yet they are absent from the “choice”. This is a key limitation in DESTINY-Breast06. The control arm is suboptimal, and does not reflect real-life practice.
What to look at during DESTINY-Breast06 presentation
Again, “thanks” to press-release, I don’t think there is any real suspense about the main results to be presented.
What I will be really looking at is the following:
HER2-low: how they really defined it? How many patients were really excluded because of HER2 negativity after central review? How this compare with previous studies?
Informative censoring: The open-label design and the suboptimal control arm raise concerns that patients will drop out in excess and early on from the control arm. If so, patients remaining in the control arm may be more likely to experience the event, leading to informative censoring. I hope we will have data on the numbers and reasons for censored patients in both arms.
Clear description of patients’ characteristics: how many got CDK4/6, how many got previous anthracyclines, etc. (hopefully better reporting than in DESTINY-Breast-04)
A breakdown of PFS events.
Toxicity is a key concern with trastuzumab deruxtecan (see Warning Box of the FDA label below), the differences in toxicity between groups will be key.
Plus many other things… but let’s not spoil too much !
What I hope not to see
Standing ovations are a recent trend at major oncology meetings. These may be the result of genuine enthusiasm from oncologists and trialists. However, they can also be triggered by sponsors with purely marketing intentions. As a community, we should be aware of this risk and try our best to avoid being manipulated by industrial interests, focusing on what truly matters to patients.
Given the limitations of the trial design I described, I hope there won’t be a standing ovation after the DESTINY-Breast06 trial presentation.
(ASCO2022 standing ovation for DESTINY-Breast04)